The Gleason score uses a scale from 1 to 5 to classify prostate tissue (see Figure 1). It is used to evaluate individual biopsy cores or a prostate specimen after prostatectomy by pathologists using dedicated microscopes.
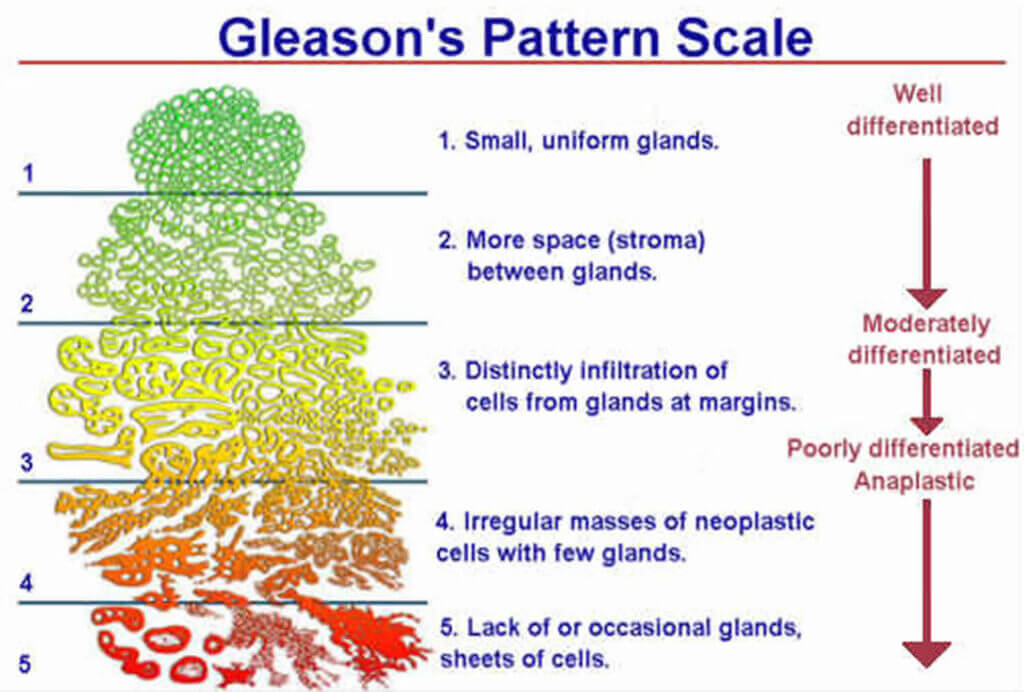
Figure 1: Schematic diagram of the Gleason score for tissue classification
The standard approach to reporting the Gleason score is to give an x+y score where x is the pattern most present in the sample (e.g., a biopsy core) and y the second most present pattern.
For instance, a Gleason score of 3+3 means that only Gleason pattern 3 is present in the sample.
A Gleason score of 3+4 means that pattern 3 is dominant in the sample, but some pattern 4 is also identified. If pattern 4 is dominant over pattern 3, the Gleason score would be 4+3, etc.
The Gleason score may sometimes be reported by the sum total: Gleason score 6 for a 3+3, Gleason score 7 for a 3+4 or 4+3, etc.
With the results of the clinical studies of the last decades, a Gleason score of 1 or 2 is no longer considered cancer. Therefore, the pattern in the case of cancer is scored from 3 to 5, and the total Gleason score ranges from 6 to 10.
The ISUP Grade Group Classification
In November 2014, the International Society of Urological Pathology (ISUP) convened to ratify an update to the system used to classify prostate cancer.
The experts thus approved a new classification method, which is clearer and easier for patients to understand, known as “ISUP Grade Groups.” This scale still relies on the Gleason score tissue classification but is now made up of levels 1-5 (see Table 1).
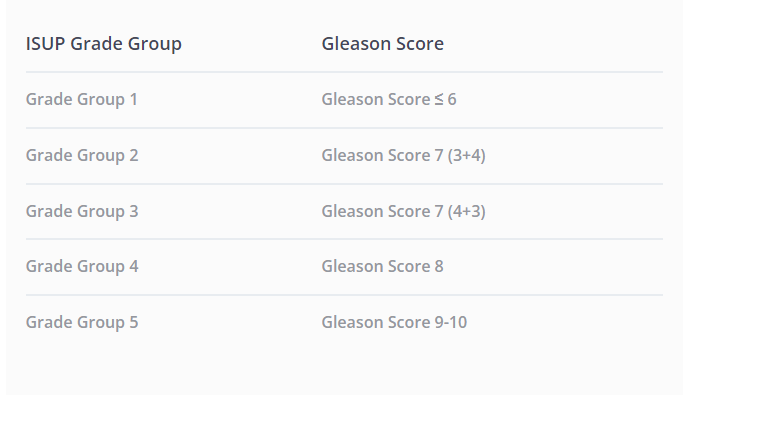
Table 1: Equivalence between the ISUP Grade Group and the historical Gleason Score classifications
The importance of the Gleason Score
While PSA testing, Digital Rectal Examination (DRE), or MRI can give a probability of a cancer diagnosis or estimate its aggressiveness, a prostate biopsy is the only medical procedure that can truly confirm a cancer diagnosis. The tissue samples obtained by the urologist during this procedure, called biopsy cores, are sent to the lab and analyzed by a pathologist, who then issues a report.
The more tissue samples (cores) taken during the biopsy, the more information the urologist will have about the patient’s prostate. However, sampling too many cores may result in oversampling, diagnosis of non-significant prostate cancer, increased risk of post-biopsy sepsis, and/or urinary retention.
Generally, 12 cores are systematically taken from the prostate, with an additional 2-3 cores targeted in suspicious areas of the MRI, if any (see Figure 2).
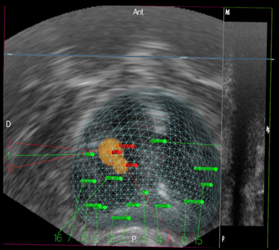
Figure 2: Example of transperineal targeted and systematic prostate biopsy with KOELIS Trinity®. MRI targets are in yellow, positive cores (cancer) in red, negative cores in green 1

The risk group directly impacts the treatment strategy that will be preferred by the urologist.
Drawbacks of the existing diagnostic methods
The potential drawbacks of conventional 2D ultrasound biopsy sampling are (see Figure 3. a, b and c):
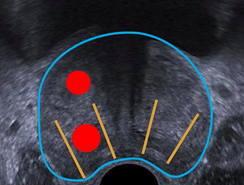
a) The biopsy misses the cancer
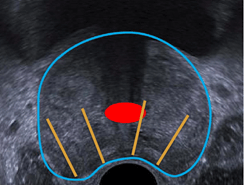
b) The biopsy undersamples a clinically significant tumor, thus underestimating the patient's actual risk group and preventing a reliable treatment strategy
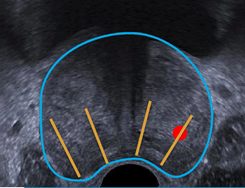
c) The biopsy oversamples a non-clinically significant tumor, which may lead to overestimating the cancer stage and overtreating the patient out of caution
With the advent of MRI, systems capable of fusing MRI with ultrasound partially solved those issues. However, conventional MRI/US fusion systems only use rigid fusion technologies that may display the MRI target in a different location than it actually is, giving the urologist a false sense of sampling the suspect area.
How KOELIS® improves prostate cancer diagnosis
The KOELIS Trinity® Prostate Fusion Biopsy System incorporates all of the exclusive technologies to deliver an accurate diagnosis, traceability, and treatment decision during prostate biopsy.
It reconstructs a dedicated 3D map of the prostate for each patient containing the prostate contour, MRI suspicious areas, and biopsy cores (see Figure 2).
The patented OBT Fusion® technology ensures a millimetric accuracy of all the 3D map information, thanks to its unique capability to compensate for prostate deformations and patient movements during the biopsy.
Combined with MRI/US elastic fusion and 3D ultrasound, OBT Fusion® enables taking the prostate’s systematic and targeted cores in a very precise way through a transperineal or transrectal approach, therefore allowing an accurate staging of the prostate and a patient-tailored treatment decision.
 United States
United States
